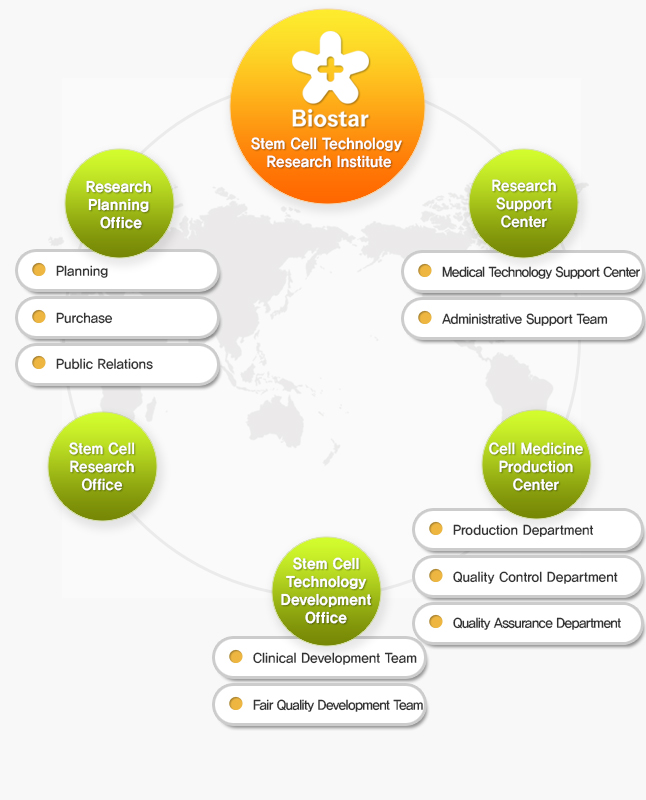
Biostar Stem Cell Technology Research Institute has the most advanced equipment for cell cultivation. It boasts production capacity of 30,000 cases or above, one of the highest in the world. Biostar Stem Cell Technology Research Institute complies with the GMP Facility Standards, the most widely followed standard for pharmaceutical production facilities, to ensure safe production.

Biostar Stem Cell Technology Research Institute has the largest separation, cultivation and storage facilities in South Korea.
- South Korea’s largest stem cell research institute
- Has the most advanced equipment to separate, cultivate and store stem cells
- Location: Gasan Digital Complex, Seoul
- Total Area: 1,421m²
- Cleanness: Class 100~100,000
All production stages are standardized, from the reception of adipose tissue to separation, cultivation and storage of stem cells. We ensure the stability of our stem cells through gene tests and other quality tests. Therefore, our stem cells retain their characteristics and cell conditions even after they have been stored for a long time until the time when they are needed. This is made possible by the special technologies applied to the standardized stem cell bank operation system of Biostar Stem Cell Technology Research Institute.

















Most Commented