Dr. Ra Jeong-chan’s research team at Biostar Stem Cell Research Institute, opens a new path to treatment of autoimmune diseases with intravenous injections of autologous adipose-derived stem cells
- Obtained approval for stem cell treatment of autoimmune diseases from the Ministry of Health, Labour and Welfare of Japan
- Official acknowledgement of the safety of intravenous administration of cultured autologous adipose-derived stem cells
- Great news for global patients with autoimmune diseases including rheumatoid arthritis, atopic dermatitis and multiple sclerosis
The era of treating autoimmune diseases with intravenous injections of autologous adipose-derived stem cells that are cultured using the patented technology of Korea has opened in Japan.
The Biostar Stem Cell Research Institute (President Dr. Ra Jeong-chan), jointly run by Nature Cell and R Bio, announced on November 27 that Nishihara Clinic, a partner medical center in Japan, had acquired approval from the Ministry of Health, Labour and Welfare of Japan to treat autoimmune diseases with intravenous injections of autologous adipose-derived stem cells.
This approval has is significant in that it recognizes the safety of intravenously administering patients’ own adipose-derived stem cells that are cultured with the patented technology of Biostar based in Korea as well as the possibility of treating autoimmune diseases including rheumatoid arthritis, atopic dermatitis and multiple sclerosis.
Dr. Ra Jeong-chan’s research team, who became the world’s first to practically use the medical technique of culturing autologous adipose-derived stem cells to treat intractable diseases through intravenous injections in 2008, currently has 94 related patents including the method of manufacturing stem cells appropriate for intravenous administration and published some 10 research papers on intravenous administration and autoimmune diseases.
The safety of intravenous administration of autologous adipose-derived stem cells to humans and animals has been confirmed through research, the results of which have been published in the journal, Stem Cell Development.
In addition, Dr. Ra Jeong-chan’s research team has successfully conducted research on various types of autoimmune diseases, based on a theory that stem cells help restore the body’s homeostasis through immunomodulatory and anti-inflammatory functions, and published a number of research papers.
The human treatment cases have been reported in the Journal of Translational Medicine, a world-renowned scientific journal (Attachment 1). In a time when there was no clear solution for treatment of autoimmune diseases, this paper garnered attention from the global medicine community as it proposed a groundbreaking treatment method using stem cells and providing scientific evidence to support the approach.
Autoimmune diseases occur when the body’s immune system attacks itself. Depending on the area and extent of the attack, it can give rise to a variety of diseases such as rheumatoid arthritis, atopic dermatitis, multiple sclerosis, autoimmune inner ear disease, lupus, scleroderma and Behcet’s disease. The symptoms persist and become chronic, thereby causing permanent damage. These intractable diseases are rarely curable with the current drugs available and cause severe pain, and various adverse events can occur due to the use of steroidal and non-steroidal anti-inflammatory drugs.
According to the American Autoimmune Related Diseases Association (AARDA), there are 138 types of autoimmune diseases that have been classified to date, and there are nearly 50 million autoimmune diseases patients in the U.S.A alone. The vast majority of patients are suffering from intractable types, for which there are no adequate treatment methods available at this time. In the case of Korea, there were 895 types of diseases selected for the medical cost assistance program for rare and intractable disease patients in 2015, and a large number of them – 145, to be exact – were autoimmune diseases.
Biostar revealed that some 400 Korean and foreign autoimmune disease patients have gone abroad to receive stem cell treatment (approximately 3,500 times) using their own stem cells. A combination of such experiences of actual patients, clinical trial and research outcomes including patents and research publications have contributed to the successful acquirement of approval from the Ministry of Health, Labour and Welfare of Japan.
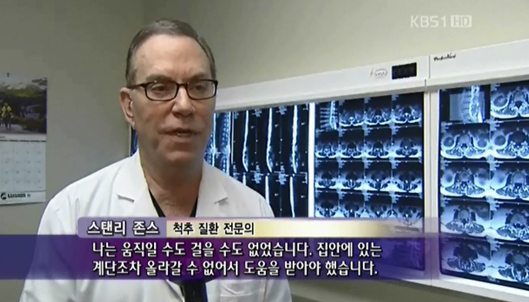
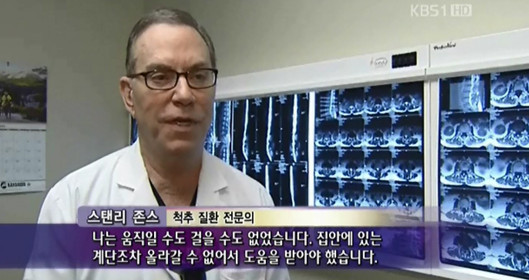
Dr. Stanley Jones, a surgeon based in Houston, U.S.A., reportedly suffered from the side effects of cortisone, a steroid medication, as he had to use increasingly larger amounts due to severe pain in his wrists and knees. After stem cell therapy, however, his symptoms improved, allowing him to perform long surgeries and cease the administration of the steroid medication and methotrexate.
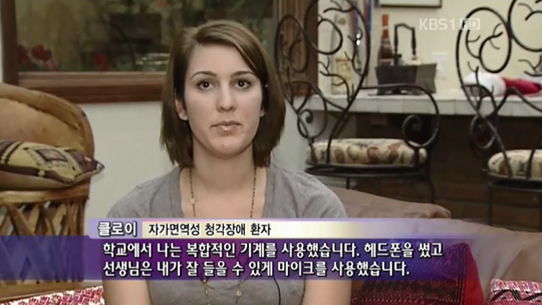
Chloe Soul, a college student in the U.S.A. who suffered from autoimmune inner ear disease, had not responded to the conventional treatment with steroidal anti-inflammatory and immunomodulatory drugs. However, her hearing improved to a normal level (scaled out to 15dB) following stem cell therapy, and she was able to pursue her dream of becoming a musical actress once again (Attachment 1. Clinical cases 6.1).
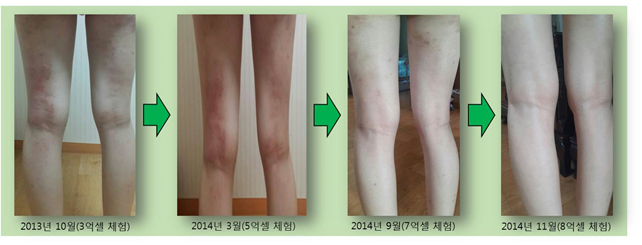
A Korean female patient, aged 19, faced difficulty in her day-to-day activities and school life as she experienced severe pain and unbearable itchiness caused by atopic syndrome. Through nine stem cell treatments, she saw a gradual improvement in her symptoms, and she is now almost completely cured, experiencing no itchiness at present.
Dr. Ra Jeong-chan, who headed the development of a stem cell treatment method involving the cultivation of autologous adipose-derived stem cells for intravenous administration, said, “It brings me joy to gain formal recognition of the safety of intravenous administration of autologous adipose-derived stem cells from the Japanese government through this approval. I am grateful to God for allowing me to do good for patients suffering from autoimmune diseases worldwide, along with those who are working toward this cause.”
Attachment 1. Biostar’s research paper (published in the Journal of Translational Medicine) with an introduction of autoimmune diseases and evidentiary data for regenerative treatment of autoimmune diseases
Attachment 2. Evidentiary data for regenerative treatment of autoimmune diseases
Attachment 3. An introduction of autoimmune diseases


Leave a reply