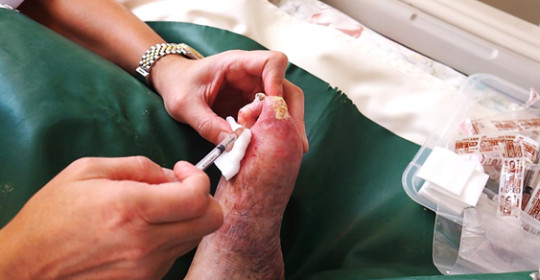
The era of regenerative medicine has begun in Japan, with the adult stem cell treatment technology developed by Biostar Stem Cell Research Institute of Korea
- The Act on Ensuring the Safety of Regenerative Medicine passed in Japan on November 25, 2015
- Treatment available for critical limb ischemia including Buerger’s disease, degenerative arthritis and skin regeneration (esthetics)
R-Japan Co., Ltd., a Japanese affiliate of Nature Cell, announced on November 25 that stem cell therapy in Japan has begun, using the adult stem cell technology developed by Korea’s Biostar Stem Cell Research Institute (President Dr. Ra Jeong-chan). In accordance with the Act on Ensuring the Safety of Regenerative Medicine, also known as the Regenerative Medicine Law, which took effect in Japan as of November 25, the processing work including the cultivation of stem cells must be implemented with the approval of the government (Ministry of Health, Labour and Welfare, MHLW), and stem cell therapy can only be administered by the medical facilities and for the diseases for which official approval has been granted by MHLW. R-Japan and Nishihara Clinic have been given official approval from MHLW of Japan, with the technology and related data obtained from Biostar, and this will open a new era of stem cell therapy, which will save lives in Japan, Korea and other parts of the world. The aggressive efforts put forth by the Abe administration of Japan to innovate the related healthcare system and lead the field of regenerative medicine will benefit countless intractable disease patients worldwide.
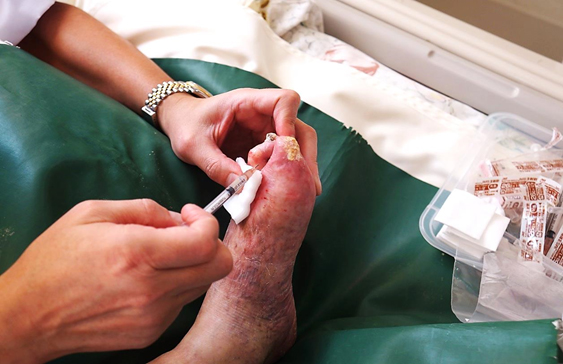
At Nishihara Clinic in Japan, Lee Seong-hee, the Chairman of the Buerger’s Disease Patients Association, is currently receiving treatment for necrosis in his feet, with injections of his own stem cells.
Lee, aged 67, who is receiving stem cell therapy in Japan, said, “I am grateful to receive treatment in Japan, with the stem cell treatment technology developed in Korea, but at the same time, it is unfortunate [that I cannot receive treatment in Korea].”
Kim Yeon-ha, aged 46, who received treatment for degenerative arthritis, said, “I’ve been postponing treatment due to lack of trust in the knee treatment procedures available in Korea before finally deciding to receive treatment in Japan where approval was given. So far, I am satisfied with the specialized system of the approved medical clinic, high-quality medical care provided by the doctors, follow-up measures, and so on.”






Leave a reply