
Global Interest is High for Biostar’s Stem Cell Treatment of Parkinson’s Disease
-Biostar StemCell Research Institute’s stem cell treatment for Parkinson’s disease is increasing in global attention post Japan’s regulatory approval
-Kudo Clinic in Tokyo signed on management of Parkinson’s disease patients through the effectiveness, safety, and continuous evaluation of stem cell treatment
Upon obtaining regulatory approval for the treatment of Parkinson’s disease from the Japanese Ministry of Health and Welfare, treatment of Parkinson’s disease has begun. A worldwide interest in this treatment technology developed by Biostar StemCell Research Institute has been growing.
The number of inquiries on this treatment is increasing not only from Asia, but also from India, the United States, Africa, and Israel. Patients are currently traveling from the United States, Turkey, and the United Kingdom to Shinjuku Clinic in Tokyo, Japan to receive treatment for Parkinson’s disease.
Accordingly, Biostar StemCell Research Institute and its affiliate, JASC (Japan Angel StemCell) have decided to improve patient care services to raise medical services on a global level. JASC has signed a contract with Kudo Clinic to establish a patient-centered treatment environment.
Kudo Clinic is a neurosurgery clinic that was first operated by Dr. Chiaki Kudo who is a neurosurgeon. The clinic has been a specialized treatment institution for patients with brain diseases such as Parkinson’s disease, dementia, and migraines since 2001.
Professional patient management medical services will be provided at Kudo Clinic. These medical services will be examined through brain MRI, MDS-UPDRS (Unified Parkinson’s Disease Rating Scale), and neurological examinations, which consistently evaluate the safety and efficacy of intravenous and intrathecal injections of stem cells. These methods are expected to play an important role in building a patient-centered treatment environment.
These medical services are expected not only to provide new hope for patients with Parkinson’s disease but also to position themselves as the model practices for medical innovation and disease management in stem cell therapy worldwide.
Based on the treatment experience in Japan, Biostar StemCell Research Institute, together with Nature Cell Co. Ltd., plans to prepare for the commercialization of advanced regenerative medical treatment technology for Parkinson’s disease patients in Korea and launch the services in Korea by 2025.

Kudo Clinic in Tokyo
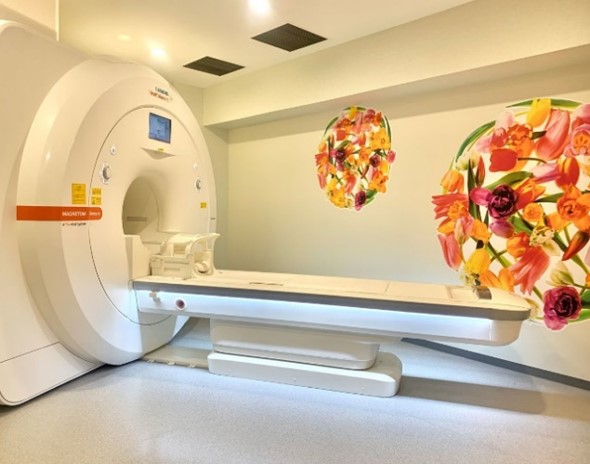
MRI exam room inside Kudo Clinic
Leave a reply




Leave a reply